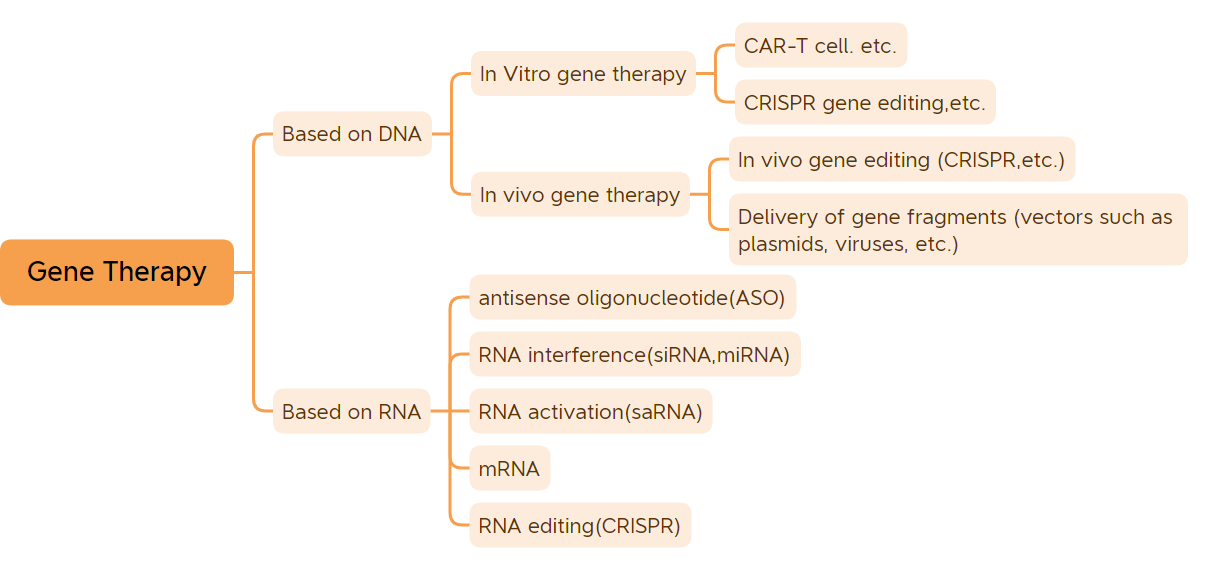
Hidde-sideyaashu waa unugyada hidde-sidaha aasaasiga ah ee xakameeya sifooyinka.Marka laga reebo hidda-wadaha fayrasyada qaarkood, kuwaas oo ka kooban RNA, hidde-sideyaasha noolaha intooda badan waxay ka kooban yihiin DNA..Inta badan cudurada noolaha waxaa sababa isdhexgalka ka dhexeeya hidde-sideyaasha iyo deegaanka.Daaweynta hidda-wadaha asal ahaan waxay daweyn kartaa ama yareyn kartaa cudurro badan.Daaweynta hidda-wadaha waxaa loo arkaa inay tahay kacaan dhanka daawada iyo farmashiyaha.Daawooyinka daawaynta hidda-wadaha ee dareen ballaadhan waxa ka mid ah iyada oo lagu salaynayo dawooyinka DNA-ga wax laga beddelay (sida dawooyinka ku-daweynta hidde-sidaha ee vivo ee ku salaysan fayraska, dawooyinka daawaynta hidda-wadaha ee vitro, daawooyinka plasmid-ka qaawan, iwm.) iyo dawooyinka RNA (sida dawooyinka oligonucleotide antisense, daawooyinka siRNA, iyo daawaynta hidda-sidaha mRNA, iwm.);dareenka cidhiidhiga ah Daawooyinka daawaynta hidda-wadaha inta badan waxa ka mid ah dawooyinka plasmid DNA-ga, dawooyinka therapy hidda-wadaha ee ku salaysan fayraska, dawooyinka daawaynta hidda-wadaha ee ku salaysan fayrasyada bakteeriyada, hababka tafatirka hidda-wadaha, iyo dawooyinka daawaynta unugyada ee wax ka beddelka hidda-wadaha in vitro.Ka dib sannado badan oo korriin jireed ah, daawaynta hidda-wadaha waxay gaadheen natiijooyin caafimaad oo dhiirigelinaya.(Lama tirin karo tallaalada DNA-ga iyo tallaallada mRNA) Waqtigan xaadirka ah, 45 dawooyinka daawaynta hidda-wadaha ayaa loo oggolaaday suuqgeynta adduunka.Wadarta 9 daawaynta hidda-wadaha ayaa loo oggolaaday suuqgeynta sanadkan, oo ay ku jiraan 7-daawaynta hidda-sidaha ee loo oggolaaday suuqgeynta markii ugu horreysay sanadkan, kuwaas oo kala ah: CARVYKTI, Amvuttra, Upstaza, Roctavian, Hemgenix, Adstiladrin iyo Ebvallo, Suuqgeynta gudaha Mareykanka Agoosto 2022, waxaana ogolaaday suuqgeyn Midowga Yurub sanadka 2019;
Kala soocida daaweynta hidda-wadaha (Isha sawirka: Bio-Matrix)
Maqaalkani waxa uu taxayaa 45 daawaynta hidda-wadaha (marka laga reebo tallaallada DNA-da iyo tallaallada mRNA) ee loo oggolaaday suuq-geyn.
1. Daaweynta hidda-wadaha ee vitro
(1) Strimvelis
Shirkadda: Waxaa soo saartay GlaxoSmithKline (GSK).
Waqtiga la suuqgeyn karo: Waxaa u oggolaaday suuqgeyn Midowga Yurub bishii Maajo 2016.
Tilmaamaha: Loogu talagalay daawaynta difaac la'aanta isku-darka daran (SCID).
Faallooyinka: Habka guud ee daawayntan waa in marka hore la helo unugyada tarma ee hematopoietic ee bukaanka, la balaadhiyo oo lagu dhaqo gudaha vitro, ka dibna la isticmaalo retrovirus si ay u soo bandhigto nuqul ka mid ah hidda-wadaha ADA (adenosine deaminase) ee unugyada tarma ee hematopoietic, oo ugu dambeyntii la isku duro unugyada tarma ee Hematopoietic ee la beddelay ayaa dib loogu soo celiyay jirka.Natiijooyinka caafimaadku waxay muujinayaan in heerka badbaadada 3-sano ee bukaannada ADA-SCID ee lagu daweeyay Strimvelis ay tahay 100%.
(2) Zalmoxis
Shirkadda: Waxaa soo saartay Shirkadda Talyaaniga MolMed.
Waqtiga suuqa: Ogolaanshaha suuq-geynta shuruudda ah ee laga helay Midowga Yurub 2016.
Tilmaamaha: Waxaa loo isticmaalaa daaweynta adjuvant ee habka difaaca jirka ee bukaanada ka dib tallaalka unugyada tarma ee hematopoietic.
Fiiro gaar ah: Zalmoxis waa unug-garabeed allogeneic T-ga is-dilka hidde-socodka difaaca jirka oo ay beddeleen vectors retroviral.Habkani waxa uu isticmaalaa fayraska retroviral si ay u habeeyaan unugyada allogeneic T unugyada, si ay unugyada hidde modified T unugyada muujiyaan 1NGFR iyo HSV-TK Mut2 hiddo-is-dilitaanka dadka u ogolaanaya in ay isticmaalaan daawooyinka ganciclovir (ganciclovir) waqti kasta si ay u dilaan T unugyada kuwaas oo sababa falcelin difaaca lidka ah, ka hortagga suurtogalka ah sii xumaansho dheeraad ah ee bukaanada GVHD .
(3) Invossa-K
Shirkadda: Waxaa soo saaray TissueGene (KolonTissueGene).
Waqtiga la suuqgeyn karo: Waa la oggolaaday liiska Koonfurta Kuuriya Luulyo 2017.
Tilmaamaha: Loogu talagalay daawaynta arthritis-ka jilibka ee xumaaday.
Xusuusin: Invossa-K waa daaweynta hidde-sideyaasha unugyada allogeneic ee ku lug leh chondrocytes aadanaha.Unugyada allogeneic waxaa lagu beddelaa hidde ahaan in vitro, unugyada la beddelayna waxay muujin karaan oo ay qarin karaan isbeddelka isbeddelka koritaanka β1 (TGF-β1) ka dib duritaanka intra articular.β1), si loo hagaajiyo calaamadaha osteoarthritis.Natiijooyinka caafimaadku waxay muujinayaan in Invossa-K ay si weyn u wanaajin karto arthritis-ka jilibka.Waxaa laashay 2019 Maamulka Cuntada iyo Dawooyinka Kuuriya sababtoo ah soo-saareyaashu waxay si khaldan u calaamadiyeen maaddooyinka la isticmaalay.
(4) Zynteglo
Shirkadda: Waxaa baadhay oo soo saartay bluebird bio.
Waqtiga la suuqgeyn karo: Waxaa ansixiyay Midowga Yurub suuqgeyntiisa 2019, oo ay ansixisay FDA ee suuqgeynta gudaha Mareykanka Agoosto 2022.
Tilmaamaha: Daaweynta ku-tiirsanaanta β-thalassaemia.
Xusuusin: Zynteglo waa daawaynta hidda-wadaha lentiviral ee vitro taas oo soo bandhigaysa nuqul shaqeynaya hiddo-wadaha β-globin ee caadiga ah (βA-T87Q-globin) unugyada tarma ee hematopoietic ee laga qaado bukaanka iyada oo loo marayo vector lentiviral , ka dibna dib u soo celi kuwan unugyada tarma ee hematopoietic autologous autologous hematopoietic.Marka bukaanku helo hiddo-wadaha βA-T87Q-globin ee caadiga ah, waxa laga yaabaa inay soo saaraan borotiinka HbAT87Q ee caadiga ah, kaas oo si wax ku ool ah u yarayn kara ama meesha ka saari kara baahida dhiig-shubidda.Waa daawayn hal mar ah oo loogu talagalay in lagu beddelo dhiig ku shubista nolosha oo dhan iyo daawaynta cimri-dhererka bukaanka 12 sano iyo ka weyn.
(5) Skysona
Shirkadda: Waxaa baadhay oo soo saartay bluebird bio.
Waqtiga la suuqgeyn karo: Waxaa oggolaaday Midowga Yurub suuqgeyntiisa Luulyo 2021, oo ay ansixisay FDA suuq-geynta gudaha Mareykanka Sebtembar 2022.
Tilmaamaha: Daawaynta hore ee adrenoleukodystrophy cerebral (CALD).
Xusuusin: Daaweynta hidda-wadaha Skysona waa daawaynta hidda-wadaha hal mar kaliya ee loo oggolaaday daawaynta adrenoleukodystrophy-cerebral-ka hore (CALD).Skysona (elivaldogene autotemcel, Lenti-D) waa unug hematopoietic stem lentiviral in vitro therapy therapy Lenti-D.Habka guud ee daawadu waa sida soo socota: Unugyada tarma ee hematopoietic autologous ayaa laga soo saaray bukaanka, beddeleen oo wax ka beddeleen lentivirus oo sidda hidda-wadaha ABCD1 ee bini'aadamka in vitro, ka dibna dib loogu celiyo bukaanka.Waxaa loo isticmaalaa in lagu daweeyo bukaannada da'doodu ka yar tahay 18 jir, sidda ABCD1 isbeddellada hidda-wadaha, iyo CALD.
(6) Kymriah
SHIRKADDA: Waxaa soo saaray Novartis.
Waqtiga suuqa: Waxaa ogolaatay FDA suuqgeyntooda Agoosto 2017.
Tilmaamaha: Daawaynta hore ee unugyada B-cell acute lymphoblastic leukemia (ALL) iyo soo noqoshada iyo dib u soo noqoshada DLBCL.
Faallooyinka: Kymriah waa daawada lentiviral in vitro hiddo-wadaha therapy, daawaynta CAR-T ee ugu horraysa ee loo oggolaaday suuqgeynta adduunka, lagu beegsanayo CD19, iyo adeegsiga 4-1BB co-stimulatory factor.Waxaa lagu qiimeeyaa $475,000 gudaha Mareykanka iyo $313,000 gudaha Japan.
(7)Yescarta
Shirkadda: Waxaa soo saartay Kite Pharma, oo hoos timaada Gilecaad (GILD).
Waqtiga suuqa: Waxaa ogolaatay FDA suuqgeyntooda Oktoobar 2017;Fosun Kite waxay soo bandhigtay tignoolajiyada Yescarta ee Kite Pharma waxayna ku soo saartay Shiinaha ka dib markii ay heshay oggolaansho.Loo ansixiyay liiska dalka.
Tilmaamaha: Daawaynta soo laabashada ama refractory lymfoma B-cell weyn.
Faallooyinka: Yescarta waa daawaynta hidda-sidaha ee dib-u-dhiska vitro, taas oo ah daawaynta CAR-T ee labaad ee la oggolaaday ee adduunka.Waxay beegsataa CD19 oo waxay qabataa kharashka CD28.Waxa lagu qiimeeyaa $373,000 gudaha Maraykanka.
(8) Tecartus
Shirkadda: Waxaa soo saartay Gilecaad (GILD).
Waqtiga suuqa: Waxa ansixisay FDA suuqgeyntiisa Luulyo 2020.
Tilmaamaha: Limfoma unugga mantle ee soo noqnoqday ama soo noqnoqda.
Faallo: Tecartus waa daawaynta unug ee CAR-T oo iskeed u gaar ah oo lagu beegsanayo CD19, waana daawaynta CAR-T ee saddexaad ee loo oggolaaday suuqgeynta adduunka.
(9) Breyanzi
SHIRKADA: Waxaa soo saartay Bristol-Myers Squibb (BMS).
Waqtiga suuqa: Waxaa ogolaatay FDA suuqgeyntooda Febraayo 2021.
Tilmaamaha: Soo noqoshada ama dib-u-celinta (R/R) lymphoma B-cell (LBCL).
Faallooyinka: Breyanzi waa daawaynta hidda-sidaha in vitro ee ku salaysan lentivirus, daawaynta afraad ee CAR-T ee loo oggolaaday suuqgeynta adduunka, laguna beegsanayo CD19.Oggolaanshaha Breyanzi ayaa guul muhiim ah u ah Bristol-Myers Squibb dhanka cilmiga difaaca unugyada, kaas oo ay heshay markii ay Celgene ku heshay $74 bilyan sanadka 2019.
(10) Abecma
Shirkadda: Waxaa iska kaashaday Bristol-Myers Squibb (BMS) iyo bluebird bio.
Waqtiga suuqa: Waxaa ogolaatay FDA suuqgeyntooda Maarso 2021.
Tilmaamaha: soo noqnoqda ama soo noqnoqda dhowr myeloma.
Faallooyin: Abecma waa daawaynta hidda-sidaha in vitro ee ku salaysan lentivirus, daawaynta unugga CAR-T ee ugu horreysa adduunka ee lagu beegsanayo BCMA, iyo daawaynta CAR-T ee shanaad ee ay ansixisay FDA.Mabda'a daawadu waa in lagu muujiyo reseptors chimeric BCMA ee unugyada T ee bukaanka iyada oo loo marayo beddelka hidda-wadaha lentivirus dhexdhexaadiyay ee gudaha vitro.Daawaynta si loo baabi'iyo unugyada T-ga ee aan hidde ahaan wax-ka-beddelin ee bukaannada, ka dibna dib loogu soo celiyo unugyada T la beddelay, kuwaas oo raadiya oo dilaaya BCMA-muujinta unugyada kansarka ee bukaannada.
(11) Libmeldy
SHIRKADA: Waxaa soo saartay Orchard Therapeutics.
Waqtiga suuqa: Waxaa ogolaaday Midowga Yurub liiska Diseembar 2020.
Tilmaamaha: Loogu talagalay daawaynta leukodystrophy metachromatic (MLD).
Faallooyinka: Libmeldy waa daawayn hidde-siyeed ku salaysan unugyo CD34+ ah oo iskood isu beddelay oo lentivirus ku beddelay vitro.Xogta caafimaadku waxay muujinaysaa in faleebo hal xidid ah oo Libmeldy ah ay si wax ku ool ah u beddeli karto koorsada bilawga hore ee MLD marka la barbar dhigo mootada daran iyo cilladda garashada ee bukaannada aan la daweyn ee isku da'da ah.
(12) Benoda
Shirkadda: Waxaa soo saaray WuXi Giant Nuo.
Waqtiga suuqa: Si rasmi ah u ansixisay NMPA Sebtembar 2021.
Tilmaamaha: Daawaynta bukaanada qaangaarka ah ee soo noqnoqday ama dib u celinaya lymphoma weyn ee B-cell (r/r LBCL) ka dib markii daawaynta nidaamka labaad ama ka sarreeya.
Faallo: Beinoda waa daawaynta hidda-sidaha CD19 ee CAR-T, sidoo kale waa badeecada xudunta u ah Shirkadda WuXi Juro.Waa sheyga labaad ee gawaarida ee lagu ansixiyay Shiinaha, marka laga reebo dib-u-dhiska / lymphoid WUXI GROWN NIOSPOMENS (MCLOCLIC LYMPHEMIA (CLL), LIMLOCLE LYMOCES (DLL), oymphoblassia neyfloblassia (oo dhan).
(13) CARVYKTI
Shirkadda: Legend Biotech alaabtii ugu horreysay ee loo oggolaaday suuqgeyn.
Waqtiga suuqa: Waxaa ogolaatay FDA suuqgeyntooda Febraayo 2022.
Tilmaamaha: loogu talagalay daawaynta soo noqoshada ama dib u laabashada Multiple myeloma (R/R MM).
Fiiro gaar ah: CARVYKTI (ciltacabtagene autoleucel, Cilta-cel si kooban) waa daawaynta difaaca unugga CAR-T oo leh laba unug-hal-domain ka-hortagga unugyada B-cell maturation antigen (BCMA).Xogtu waxay muujinaysaa in CARVYKTI Bukaannada qaba myeloma badan oo soo noqnoqday ama dib u celinaya kuwaas oo helay afar ama in ka badan oo daaweyn ah (oo ay ku jiraan proteasome inhibitors, immunomodulators iyo anti-CD38 monoclonal antibodies), heerka jawaabta guud ee 98% ayaa la muujiyay.
(14)Ebvallo
SHIRKADA: Waxaa soo saartay Atara Biotherapeutics.
Komishanka Yurub (EC) ee suuq-geynta Diisambar 2022, waa daawaynta unugyada T unu ee ugu horreeya adduunka ee loo oggolaaday suuqgeynta.
Tilmaamaha: Sida monotherapy loogu talagalay fayraska Epstein-Barr (EBV) ee la xidhiidha ka-tallaalidda lymfoproliferative ka dib (EBV+PTLD), bukaannada helaya daawaynta waa inay ahaadaan dad waaweyn iyo carruur ka weyn 2 sano oo hore u helay ugu yaraan hal daaweyn kale oo daroogo ah.
Faallooyinka: Ebvallo waa daawaynta hidda-wadaha T-unugyada caalamiga ah ee gaarka ah ee EBV-ga kaas oo bartilmaameedsada oo meesha ka saaraya unugyada EBV-samay hab xaddidan HLA.Oggolaanshaha daawayntan waxay ku salaysan tahay natiijooyinka daraasadda tijaabada ah ee wajiga 3aad ee muhiimka ah, natiijaduna waxay muujisay in ORR ee kooxda HCT iyo kooxda SOT ay ahaayeen 50%.Sicirka cafiska oo dhamaystiran (CR) wuxuu ahaa 26.3%, heerka qayb cafinta (PR) wuxuu ahaa 23.7%, wakhtiga dhexe ee cafiska (TTR) wuxuu ahaa 1.1 bilood.Bukaannada 19 ee helay cafiska, 11 ka mid ah waxay lahaayeen muddo jawaab celin ah (DOR) oo ka badan 6 bilood.Intaa waxaa dheer, marka la eego badbaadada, ma dhicin falcelin xun sida cudurka tallaalka-ka-horjeedka-martida (GvHD) ama cilladda sii-deynta cytokine ee la xiriirta Ebvallo.
2. Daaweynta hidda-wadaha ee Vivo oo ku salaysan fayraska
(1) Jinsiga/Jin Sheng
Shirkadda: Waxaa soo saartay Shirkadda Shenzhen Saibainuo.
Waqtiga suuqa: La oggolaaday in lagu taxay Shiinaha 2003.
Tilmaamaha: Daawaynta Kansarka unugga squamous-ka ee madaxa iyo qoorta.
Fiiro gaar ah: Recombinant human p53 cirbadeynta adenovirus Gendicine/Jinyousheng waa daawaynta hidda-sidaha adenovirus oo leh xuquuq madax-banaan oo hanti garaadka ay leedahay Shirkadda Shenzhen Saibainuo.Nooca aadanaha 5 adenovirus wuxuu ka kooban yahay adenovirus aadanaha nooca 5. Kan hore waa qaab-dhismeedka ugu muhiimsan ee saamaynta anti-buro ee daroogada, iyo kan dambe inta badan u dhaqmo sida side.Cudurka adenovirus wuxuu qaadaa hidda-wadaha p53 ee daweynta unugga bartilmaameedka, wuxuu muujiyaa hidda-wadaha burooyinka p53 ee unugyada bartilmaameedka, iyo muujinta hidda-wadaha Alaabta ayaa kor u qaadi karta noocyo kala duwan oo hiddo-wade-ka-hortagga kansarka ah waxayna hoos u dhigtaa hawlaha noocyada kala duwan ee oncogenes, taas oo kor u qaadeysa burooyinka jirka ee baabi'inta saameynta burooyinka iyo dilka.
(2) Rigvir
Shirkadda: Waxaa soo saartay Shirkadda Latima, Latvia.
Waqtiga liiska: Waa la oggolaaday liiska Latvia 2004.
Tilmaamaha: Daawaynta melanoma.
Fiiro gaar ah: Rigvir waa daawayn hidde-siyeed ku salaysan hidde ahaan wax laga beddelay ECHO-7 fayraska enterovirus.Waqtigan xaadirka ah, dawada waxaa lagu qaatay Latvia, Estonia, Poland, Armenia, Belarus, iwm.Kiisaska kiliinikada ee tobankii sano ee la soo dhaafay waxay caddeeyeen in fayraska Rigvir oncolytic uu yahay badbaado iyo waxtar leh, wuxuuna kordhin karaa heerka badbaadada bukaanka melanoma 4-6 jeer.Intaa waxaa dheer, daawayntan ayaa sidoo kale lagu dabaqi karaa noocyo kala duwan oo kansar ah, oo ay ku jiraan kansarka mindhicirka, kansarka ganaca, kansarka kaadiheysta, kansarka kelyaha, kansarka qanjirka 'prostate', kansarka sambabada, kansarka ilmo-galeenka, lymphosarcoma, iwm.
(3) Kansarka
Shirkadda: Waxaa soo saartay Shanghai Sanwei Biological Company.
Waqtiga suuqa: La oggolaaday in lagu taxay Shiinaha 2005.
Tilmaamaha: daaweynta burooyinka madaxa iyo qoorta, kansarka beerka, kansarka ganaca, kansarka ilmo-galeenka iyo kansarrada kale.
Xusuusin: Oncorine (安科瑞) waa shey daawaynta hidda-wadaha fayraska oncolytic iyadoo la isticmaalayo adenovirus sida side.Adenovirus oncolytic ayaa la helay, kaas oo si gaar ah ugu soo celin kara p53 hiddo-wadaha cilladaysan ama burooyinka aan caadiga ahayn, taasoo keenta lysis unugyada burooyinka, oo dila unugyada burooyinka.iyada oo aan waxyeello u geysan unugyada caadiga ah.Daraasadaha caafimaadku waxay muujiyeen in Ankerui uu leeyahay badbaado wanaagsan iyo waxtarka noocyada kala duwan ee burooyinka halista ah.
(4) Glybera
Shirkadda: Waxaa soo saartay uniQure.
Waqtiga suuqa: La ansixiyay liiska Yurub 2012.
Tilmaamaha: Daawaynta lipoprotein lipase deficiency (LPLD) oo leh xaalado daran ama soo noqnoqda ee pankreatit inkasta oo ay jirto cunto dufan ah oo si adag loo xaddiday.
Faallooyinka: Glybera (alipogene tiparvovec) waa daawaynta hidda-socodka ee ku salaysan AAV, taas oo u adeegsata AAV sida side si ay u beddesho hidda-socodka daweynta LPL unugyada muruqa, si unugyada u dhigma ay soo saari karaan qadar gaar ah oo lipoprotein lipase ah, Si loo yareeyo cudurka, daawayntani waxay waxtar u leedahay muddo dheer (maareyn sanado badan).Daawada ayaa laga saaray suuqa 2017. Sababta ka bixitaankeeda waxay la xiriiri kartaa laba arrimood: qiimaha sare iyo baahida suuqa xaddidan.Celcelis ahaan kharashka daawaynta ee daawadu waxay gaaraysaa US$1 milyan, hal bukaan oo kaliya ayaa iibsaday oo isticmaalay ilaa hadda.Inkasta oo shirkadda caymiska caafimaadku ay u soo celisay US$900,000, haddana culays wayn ayay ku tahay shirkadda caymiska.Intaa waxaa dheer, calaamadaha lagu beegsanayo daroogada ayaa ah kuwo aad dhif u ah, iyadoo heerka dhacdooyinka ee ku saabsan 1 ee 1 milyan iyo heerka sare ee ogaanshaha khaldan.
(5) Macaan
Shirkadda: Waxaa soo saaray Amgen.
Waqtiga suuqa: 2015, waxaa la ansixiyay in lagu daro Maraykanka iyo Midowga Yurub.
Tilmaamaha: Daawaynta nabarrada melanoma ee aan si buuxda looga saari karin qaliinka.
Fayras: Imlygic waa fayraska fayraska herpes simplex ah oo la yareeyey nooca 1 kaas oo lagu beddelay tignoolajiyada hidde-sideyaasha (la tirtiray jajabyada hidda-wadaha ICP34.5 iyo ICP47, iyo gelinta unugyada granulocyte macrophage colony-stimulating factor GM-CSF ee fayraska) (HSV-1) fayraska oncolytic waa fayraska hiddo-wadaha ee FDA-app ugu horreeyaHabka maamulku waa cirbadeynta intralesional, taas oo si toos ah loogu duri karo nabarrada melanoma si ay u keento dillaaca unugyada burooyinka, sii daaya antigens-ka-soo-baxa iyo GM-CSF, iyo kor u qaadida jawaabaha difaaca-ka-hortagga buro.
(6) Luxturna
Shirkadda: Waxaa soo saartay Spark Therapeutics, oo hoos timaada Roche.
Waqtiga suuqgeynta: Waxaa loo oggolaaday suuq-geynta FDA 2017, ka dibna loo oggolaaday suuq-geynta Yurub 2018.
Tilmaamaha: Daawaynta carruurta iyo dadka waaweyn ee aragga ka lumay sababtuna tahay laba-koobi ee isbeddellada hidda-wadaha RPE65 laakiin waxay haystaan tiro ku filan oo ah unugyo indho-tiran oo shaqayn kara.
Xusuusin: Luxturna waa daawayn hidde-side-ku-salaysan AAV oo lagu maamulo duritaanka subretinal.Daaweynta hidda-wadaha waxay isticmaashaa AAV2 sida side si ay u soo bandhigto nuqul shaqeynaya oo ah hiddo-wadaha RPE65 ee caadiga ah ee unugyada retinal ee bukaanka, si unugyada u dhigma ay u muujiyaan borotiinka RPE65 ee caadiga ah, taasoo ka dhigaysa yaraanta borotiinka RPE65 ee bukaanka, taas oo hagaajinaysa aragtida bukaanka.
(7) Zolgensma
Shirkadda: Waxaa soo saartay AveXis, oo hoos timaada Novartis.
Waqtiga suuqa: Waxa ansixisay FDA suuqgeyntooda May 2019.
Tilmaamaha: Daaweynta Atrophy Muscular Atrophy (SMA) bukaanka ka yar 2 sano.
Xusuusin: Zolgensma waa daawayn hidde-side ku salaysan AAV vector.Daawadani waa qorshaha kaliya ee daawaynta hal mar ah ee atrophy muruqa laf dhabarta ee loo oggolaaday suuqgeynta adduunka.Bilawga daawadu waxay fureysaa xilli cusub oo lagu daaweynayo atrophy muruqa laf dhabarta.bogga, waa horumar taariikhi ah.Daaweynta hidda-wadaha Tani waxay isticmaashaa vector scAAV9 si ay u soo bandhigto hidda-wadaha SMN1 ee caadiga ah bukaanka iyada oo loo marayo faleebo xididka si loo soo saaro borotiinka caadiga ah ee SMN1, taas oo kor u qaadeysa shaqada unugyada ay saameeyeen sida dareemayaasha dhaqdhaqaaqa.Taas bedelkeeda, dawooyinka SMA Spinraza iyo Evrysdi waxay u baahan yihiin qiyaaso soo noqnoqda oo muddo dheer ah.Spinraza waxaa lagu duraa laf dhabarta afartii biloodba mar, Evrysdina waa daawo afka laga qaato oo maalinle ah.
(8) Daalimiin
Shirkadda: Waxaa soo saaray Daiichi Sankyo Company Limited (TYO: 4568).
Waqtiga suuqa: Oggolaanshaha shuruuda ah ee ka yimid Wasaaradda Caafimaadka, Shaqada iyo Daryeelka ee Japan (MHLW) ee Juun 2021.
Tilmaamaha: Daawaynta glioma malignant.
Faallooyin: Delytact waa badeecada afraad ee daawaynta hidda-wadaha fayraska oncolytic ee la ansixiyey adduunka oo dhan, iyo badeecadii ugu horreysay ee fayraska oncolytic ee loo oggolaaday daawaynta glioma malignant.Delytact waa fayraska loo yaqaan 'herpes simplex virus type 1' (HSV-1) oo ay soo saareen Dr. Todo iyo asxaabtiisa.Delytact waxay soo bandhigaysaa isbeddellada tirtirka dheeraadka ah ee G207 genome ee jiilka labaad ee HSV-1, iyada oo kor u qaadaysa ku-noqoshadeeda xulashada unugyada kansarka iyo soo-saarka jawaabaha difaaca-ka-hortagga buro iyadoo la ilaalinayo badbaadada sare.Delytact waa jiilka saddexaad ee oncolytic HSV-1 oo hadda ku socda qiimayn caafimaad.Oggolaanshaha Delytact ee Japan waxay inta badan ku salaysan tahay tijaabada caafimaadka wajiga 2 ee gacantaBukaannada qaba glioblastoma oo soo noqnoqda, Delytact waxay gaadhay barta ugu dambaysa ee heerka badbaadada hal sano, natiijaduna waxay muujisay in Delytact ay muujisay waxtarka wanaagsan marka loo eego G207.Xoog taranka xoog leh iyo dhaqdhaqaaqa antitumor sare.Tani waxay wax ku ool u ahayd noocyada burooyinka adag ee naaska, prostate, schwannomas, nasopharyngeal, hepatocellular, colorectal, malignant burooyinka galka dareemaha ee dareemayaasha, iyo kansarka tayroodh.
(9) Upstaza
SHIRKADDA: Waxaa soo saartay PTC Therapeutics, Inc. (NASDAQ: PTCT).
Waqtiga la suuqgeyn karo: Waxaa ansixiyay Midowga Yurub suuqgeyntiisa Luulyo 2022.
Tilmaamaha: Deficiency L-amino acid decarboxylase (AADC), waxaa loo oggolaaday daawaynta bukaanka da'doodu tahay 18 bilood iyo ka weyn.
Xusuusin: Upstaza™ (eladocagene exuparvovec) waa daawaynta hidda-wadaha in vivo oo leh adeno-la-xidhiidha fayraska nooca 2 (AAV2) sida side.Bukaan-jiifku way bukoon yihiin sababtoo ah isbeddellada hidda-wadaha ee dhigaya ensaymka AADC.AAV2 waxa uu sido hiddo-wade caafimaad qaba oo codaynaya ensaymka AADC.Qaabka magdhowga hiddasidaha wuxuu gaaraa saameyn daweyn.Aragti ahaan, hal maamul ayaa waxtar leh muddo dheer.Waa daawaynta hidda-sidaha ee ugu horreysa ee la suuq geeyo oo si toos ah maskaxda loogu duro.Oggolaanshaha suuqgeyntu waxay khusaysaa dhammaan 27ka waddan ee xubnaha ka ah EU, iyo sidoo kale Iceland, Norway iyo Liechtenstein.
(10) Roctavian
Shirkadda: Waxaa soo saartay BioMarin Pharmaceutical (BioMarin).
Waqtiga la suuq-geyn karo: Waxaa oggolaaday suuq-gaynta Midowga Yurub Agoosto 2022;Oggolaanshaha suuq-geynta ee Maamulka Dawooyinka iyo Caafimaadka UK (MHRA) ee Noofambar 2022.
Tilmaamaha: Daawaynta bukaanada qaangaarka ah ee qaba hemophilia daran oo aan lahayn taariikh ka hortag FVIII factor waxayna taban u yihiin unugyada difaaca ee AAV5.
Faallooyinka: Roctavian (valoctocogene roxaparvovec) waxay u isticmaashaa AAV5 sida faleebo oo waxay isticmaashaa dhiirigeliyaha beerka gaarka ah ee HLP si uu u wado muujinta xinjirowga aadanaha VIII (FVIII) oo leh B domain la tirtiray.Go'aanka Komishanka Yurub ee lagu ansixiyay suuq-geynta valoctocogene roxaparvovec wuxuu ku salaysan yahay xogta guud ee mashruuca horumarinta bukaan-socodka daawada.Waxaa ka mid ah, natiijooyinka tijaabada bukaan-socodka ee wajiga III GENER8-1 waxay muujisay in marka la barbardhigo xogta sanadka ka hor inta aan la qorin, ka dib hal faleebo oo ah valoctocogene roxaparvovec, Maaddada heerka dhiig-baxa sanadlaha ah (ABR) ayaa si weyn hoos loogu dhigaa, inta jeer ee isticmaalka xinjirowga dhiigga recombinant VIII (F8) diyaarinta borotiinka ayaa hoos u dhacay, ama dhaqdhaqaaqa dhiigga ee F8 ayaa si weyn u kordhay.Kadib 4 todobaad oo daaweyn ah, heerka isticmaalka F8 ee maadada sanadlaha ah iyo ABR ee u baahan daawaynta ayaa la dhimay 99% iyo 84%, siday u kala horreeyaan, farqiga u dhexeeyaana wuxuu ahaa mid muhiim ah (p<0.001).Astaanta badbaadada ayaa ahayd mid wanaagsan, mana jirin mawduuc la kulmay xannibaadda F8 factor, malignant ama xinjirowga lidka ku ah, iyo dhacdooyinka xun xun ee daawaynta ee la xidhiidha (SAEs) lama soo sheegin.
(11) Hemgenix
Shirkadda: Waxaa soo saartay UniQure Corporation.
Waqtiga suuqa: Waxaa ogolaatay FDA suuqgeyntooda Noofembar 2022.
Tilmaamaha: Daawaynta bukaanka qaangaarka ah ee qaba hemophilia B.
Xusuusin: Hemgenix waa daawayn hidde-side ku salaysan AAV5.Daawadu waxay ku qalabaysan tahay factor coagulation IX (FIX) kala duwanaanshaha hidde-sideyaasha FIX-Padua, kaas oo lagu maamulo xididada.Maamulka ka dib, hiddo-wadaha ayaa muujin kara FIX xinjirowga dhiigga ee beerka iyo qarsoodiga Ka dib marka la galo dhiigga si loo sameeyo shaqada xinjirowga, si loo gaaro ujeedada daaweynta, aragti ahaan, hal maamul ayaa waxtar leh muddo dheer.
(12) Adstiladrin
Shirkadda: Waxaa soo saartay Ferring Pharmaceuticals.
Waqtiga suuqa: Waxaa ogolaatay FDA suuqgeyntooda Diseembar 2022.
Tilmaamaha: Daaweynta khatarta sare leh ee kansarka kaadiheysta aan muruqa ahayn (NMIBC) ee aan ka jawaabin Bacillus Calmette-Guerin (BCG) .
Faallooyinka: Adstiladrin waa daawayn hidde-side ah oo ku salaysan fayraska adenoviral oo aan soo noqnoqon, kaas oo si xad dhaaf ah u saari kara borotiinka interferon alfa-2b ee unugyada bartilmaameedka, waxaana lagu maamulaa tuubada kaadida ee kaadiheysta (waxaa la maamulaa hal mar saddexdii biloodba mar), fayraska fayraska wuxuu si wax ku ool ah u qaadsiin karaa unugyada derbiga kaadiheysta, ka dibna borotiinka ayaa si xad dhaaf ah u saameeya interferon.Habkan cusub ee daawaynta hidda-wadaha ayaa sidaas awgeed u beddela unugyada gidaarka kaadiheysta bukaan-socodka oo u beddela "warshad" yar oo soo saarta interferon, taas oo kor u qaadeysa awoodda bukaan-socodka ee la dagaalanka kansarka.
Badbaadada iyo waxtarka Adstiladrin ayaa lagu qiimeeyay daraasad xarun caafimaad oo badan oo ay ku jiraan 157 bukaan oo qaba halista sare ee BCG-aan ka jawaabin NMIBC.Bukaan-socodka waxay qaadanayeen Adstiladrin saddexdii biloodba mar ilaa 12 bilood, ama ilaa sunta aan la aqbali karin ee daaweynta ama soo noqoshada heerka sare ee NMIBC.Guud ahaan, boqolkiiba 51 bukaannada diiwaangashan ee lagu daweeyay Adstiladrin waxay heleen jawaab celin dhammaystiran (la'aanta dhammaan calaamadaha kansarka ee lagu arkay cystoscopy-ga, unugyada biopsy, iyo kaadida).
3. Daawooyinka yar yar ee nucleic acid
(1) Vitravene
Shirkadda: Waxaa si wadajir ah u sameeyay Ionis Pharma (oo hore u ahaan jiray Isis Pharma) iyo Novartis.
Waqtiga suuqa: 1998 iyo 1999, waxaa u ansixiyay suuqgeyn FDA iyo EU EMA.
Tilmaamaha: Daawaynta cytomegalovirus retinitis ee bukaanka HIV-ga qaba.
Faallooyinka: Vitravene waa daawada oligonucleotide antisense, taas oo ah daawada oligonucleotide ee ugu horreysa ee loo oggolaaday suuqgeynta adduunka.Marxaladda bilawga ah ee liiska, baahida suuqa ee dawooyinka lidka-CMV waxay ahayd mid degdeg ah;ka dib, sababtoo ah horumarinta daaweynta antiretroviral oo aad u firfircoon, tirada kiisaska CMV ayaa si aad ah hoos ugu dhacday.Baahida suuqa oo aad u liidata awgeed, dawada waxa la bilaabay 2002 iyo 2006 ka bixitaan wadamada EU iyo Maraykanka.
(2) Macugen
Shirkadda: Waxaa iska kaashaday Pfizer iyo Eyetech.
Waqtiga la suuq-geyn karo: Waa la oggolaaday liiska Maraykanka 2004tii.
Tilmaamaha: loogu talagalay daaweynta neovascular da'da la xiriirta macular degeneration.
Faallooyinka: Macugen waa daawada oligonucleotide pegylated oo wax laga beddelay, kaas oo bartilmaameedsan kara oo xidhi kara factor koritaanka endothelial vascular (VEGF165 subtype), iyo habka maamulka waa duritaanka intravitreal.
(3) Defitelio
Shirkadda: Waxaa soo saaray Jazz Pharmaceuticals.
Waqtiga la suuqgeyn karo: Waxaa loo oggolaaday suuq-geynta Midowga Yurub 2013-kii waxaana u oggolaaday FDA suuqgeyntooda Maarso 2016.
Tilmaamaha: Daawaynta cudurka cagaarshowga veno-occlusive ee la xidhiidha kelyaha ama sambabada cilladda ka dib wareejinta unugyada tarma ee hematopoietic.
Faallooyinka: Defitelio waa daawada oligonucleotide, taas oo ah isku dhafka oligonucleotides oo leh sifooyinka plasmin.Suuqa lagala baxay 2009 sababo ganacsi awgeed.
(4) Kynamro
Shirkadda: Waxaa iska kaashaday Ionis Pharma iyo Kastle.
Waqtiga suuqa: Sannadkii 2013, waxa loo oggolaaday in lagu suuq-geeyo gudaha Maraykanka sida daroogada agoonta ah.
Tilmaamaha: Daawaynta adjuvant ee homozygous familial hypercholesterolemia.
Fiiro gaar ah: Kynamro waa daawada oligonucleotide antisense, kaas oo ah oligonucleotide-ka-hortagga ah oo bartilmaameedsanaya apo B-100 mRNA.Kynamro waxaa loo maamulaa sida 200 mg oo hoostiisa ah hal mar todobaadkii.
(5) Spinraza
Shirkadda: Waxaa soo saartay Ionis Pharmaceuticals.
Waqtiga suuqa: Waxa ansixisay FDA suuqgeyntooda Diseembar 2016.
Tilmaamaha: Daawaynta atrophy muruqa laf dhabarta (SMA).
Xusuusin: Spinraza (nusinersen) waa daawada oligonucleotide lidka ku ah.Iyadoo lagu xirayo goobta jeexjeexa ee SMN2 exon 7, Spinraza waxay bedeli kartaa kala-goynta RNA ee hidda-wadaha SMN2, taas oo kordhinaysa soosaarka borotiinka SMN oo si buuxda u shaqeeya.Bishii Agoosto 2016, BIOGEN waxay adeegsatay ikhtiyaarkeeda si ay u hesho xuquuqda caalamiga ah ee Spinraza.Spinraza kaliya waxay bilawday tijaabadii ugu horeysay ee kiliinikada ee bini'aadamka 2011. Kaliya 5 sano, waxaa ansixiyay FDA ee suuq-geynta 2016, taas oo ka tarjumaysa aqoonsiga buuxa ee FDA ee waxtarkeeda.Daawada waxaa loo oggolaaday suuqgeynta Shiinaha bishii Abriil 2019. Dhammaan wareegga oggolaanshaha ee Spinraza ee Shiinaha waxay ka yaraayeen 6 bilood, waxayna ahayd 2 sano iyo 2 bilood tan iyo markii Spinraza markii ugu horreysay lagu ansixiyay Maraykanka.Xawaaraha liiska Shiinaha ayaa durba aad u dheereeya.Tani waxay sidoo kale sabab u tahay xaqiiqda ah in Xarunta Qiimaynta Maandooriyaha ay soo saartay "Ogaysiis ku saabsan Daabacaadda Liiska Dufcaddii Koowaad ee Daawooyinka Cusub ee Dibadda ee Degdegga ah ee loogu baahan yahay Dhaqanka Kiliinikada" Noofambar 1, 2018, waxaana lagu daray dufcaddii ugu horreysay ee 40 daawo cusub oo ajnabi ah oo loogu talagalay dib-u-eegis degdeg ah, oo ay ka mid yihiin Spinraza.
(6) Fannaaniinta 51
Shirkadda: Waxaa soo saartay AVI BioPharma (oo markii dambe loo beddelay Sarepta Therapeutics).
Waqtiga suuqa: Bishii Sebtembar 2016, waxaa FDA u oggolaatay suuqgeyn.
Tilmaamaha: Daawaynta Duchenne muscular dystrophy (DMD) oo leh exon 51 oo ka boodaya isbeddelka hidda-wadaha ee hidda-wadaha DMD.
Faallooyinka: Exondys 51 waa daawada oligonucleotide antisense, oligonucleotide antisense waxay ku xidhi kartaa booska exon 51 ee pre-mRNA ee hidda-wadaha DMD, taasoo keentay samaynta mRNA baaluq, qayb ka mid ah exon 51 waa la xannibay Excision, taas oo qayb ahaan hagaajinaysa qaababka akhriska mRNA ee caadiga ah, taas oo ka caawinaysa hagaajinta qaabdhismeedka borotiinka ee mRNA. calaamadaha bukaanka.
(7) Tegsedi
Shirkadda: Waxaa soo saartay Ionis Pharmaceuticals.
Waqtiga la suuqgeyn karo: Waxaa u oggolaaday suuqgeyn Midowga Yurub bishii Luulyo 2018.
Tilmaamaha: Daawaynta amyloidosis transthyretin ee la iska dhaxlo (hATTR).
Faallooyinka: Tegsedi waa daawada oligonucleotide-ka-hortagga ah ee lagu beegsanayo transthyretin mRNA.Waa dawadii ugu horaysay ee loo ogolaado aduunka daawaynta hatTR.Waxa lagu maamulaa duritaanka subcutaneous.Daroogadu waxay yaraynaysaa soo saarista borotiinka ATTR iyadoo la beegsanayo mRNA of transthyretin (ATTR), waxayna leedahay saamiga faa'iido-khatarta wanaagsan ee daaweynta ATTR, iyo neuropathy bukaanka iyo tayada nolosha ayaa si weyn loo hagaajiyay, waxayna la jaan qaadayaan noocyada isbeddelka TTR, Heerka cudurka iyo joogitaanka wadnaha wadnaha midna ma khusayn.
(8) Onpattro
Shirkadda: Waxaa si wadajir ah u sameeyay Alnylam Corporation iyo Shirkadda Sanofi.
Waqtiga la suuqgeyn karo: Waa la oggolaaday liiska Maraykanka 2018.
Tilmaamaha: Daawaynta amyloidosis transthyretin ee la iska dhaxlo (hATTR).
Fikradaha: Onpattro waa daawada siRNA ee lagu beegsanayo transthyretin mRNA, taas oo yaraynaysa soo saarista borotiinka ATTR ee beerka waxayna yaraynaysaa kaydka amyloid ee dareemayaasha durugsan iyada oo la beegsanayo mRNA of transthyretin (ATTR), taas oo hagaajinaysa oo yaraynaysa calaamadaha cudurka.
(9) Givlaari
Shirkadda: Waxaa soo saartay Alnylam Corporation.
Waqtiga suuqa: Waxaa ogolaatay FDA suuqgeyntooda Noofambar 2019.
Tilmaamaha: Loogu talagalay daawaynta cagaarshow ba'an (AHP) ee dadka waaweyn.
Faallooyinka: Givlaari waa daawada siRNA, taas oo ah dawada labaad ee siRNA ee loo ogolaaday suuqgeyn kadib Onpattro.Habka loo maareeyo waa cirbad subcutaneous ah.Daroogadu waxay bartilmaameed ka dhigtaa mRNA ee borotiinka ALAS1, iyo daawaynta bilaha ah ee Givlaari waxay si weyn oo joogto ah u yareyn kartaa heerka ALAS1 ee beerka, taas oo yaraynaysa heerarka neurotoxic ALA iyo PBG ee heerka caadiga ah, taas oo yaraynaysa calaamadaha cudurka bukaanka.Xogta ayaa muujisay in bukaanada lagu daweeyay Givlaari ay hoos u dhaceen 74% tirada suuxdinta marka loo eego kooxda placebo.
(10) Vyondys53
SHIRKADA: Waxaa soo saartay Sarepta Therapeutics.
Waqtiga suuqa: Waxa ansixisay FDA suuqgeyntooda Diseembar 2019.
Tilmaamaha: Daawaynta bukaanada DMD ee qaba hidda-wadaha dystrophin exon 53 mutation.
Faallooyinka: Vyondys 53 waa daawada oligonucleotide antisense, kaas oo bartilmaameedsada habka kala qaybinta dystrophin pre-mRNA.Exon 53 qayb ahaan waa la jarjaray, tusaale ahaan kuma jiraan mRNA-ga qaan-gaadhka ah, waxaana loogu talagalay in lagu soo saaro dystrophin go'ay laakiin wali shaqaynaysa, taas oo kor u qaadaysa awoodda jimicsiga bukaanka.
(11) Waylivra
Shirkad: Waxaa soo saartay Ionis Pharmaceuticals iyo qaybteeda Akcea Therapeutics.
Waqtiga la suuqgeyn karo: Waxaa loo oggolaaday suuqgeyntiisa Wakaaladda Daawooyinka Yurub (EMA) bishii Maajo 2019.
Tilmaamaha: Sida daawaynta adjuvant marka lagu daro xakamaynta cuntada ee bukaanada qaangaarka ah ee qaba familial chylomicronemia syndrome (FCS).
Faallooyinka: Waylivra waa daawada oligonucleotide antisense, taas oo ah daawadii ugu horreysay ee loo oggolaaday suuqgeynta adduunka ee daaweynta FCS.
(12) Leqvio
Shirkadda: Waxaa soo saaray Novartis.
Waqtiga suuqa: Waxaa ansixiyay Midowga Yurub suuqgeyntiisa Disembar 2020.
Tilmaamaha: Daawaynta dadka waaweyn ee qaba hypercholesterolemia aasaasiga ah (heterozygous familial iyo non-familial) ama dyslipidemia isku dhafan.
Fiiro gaar ah: Leqvio waa daawada siRNA oo bar-tilmaameedsanaysa PCSK9 mRNA.Waa daawaynta siRNA ee ugu horeysay aduunka ee hoos u dhigista kolestaroolka (LDL-C).Waxa lagu maamulaa duritaanka subcutaneous.Daawadu waxay yaraynaysaa heerka borotiinka PCSK9 iyada oo loo marayo faragelinta RNA, taas oo hoos u dhigaysa heerka LDL-C.Xogta caafimaadku waxay muujinaysaa in bukaanada aan hoos u dhigi karin heerarka LDL-C ilaa heerka bartilmaameedka ka dib daawaynta qiyaasta ugu badan ee statins, Leqvio waxay hoos u dhigi kartaa LDL-C ilaa 50%.
(13)Oxlumo
Shirkadda: Waxaa soo saartay Alnylam Pharmaceuticals.
Waqtiga la suuqgeyn karo: Waxaa ansixiyay Midowga Yurub suuqgeyntiisa Noofambar 2020.
Tilmaamaha: Daaweynta hyperoxaluria aasaasiga ah nooca 1 (PH1).
Fiiro gaar ah: Oxlumo waa dawada siRNA oo bar-tilmaameedsanaysa hydroxyacid oxidase 1 (HAO1) mRNA, habka maamulkana waa duritaanka maqaarka hoostiisa.Daawada waxaa la soo saaray iyadoo la adeegsanayo kimistarigii xasilinta ee Alnylam ee ugu dambeeyay, tignoolajiyada isku xidhka ESC-GalNAc, taas oo u sahlaysa siRNA hoosteeda oo la maareeyo adkaysi iyo adkaysi.Daroogadu waxay hoos u dhigtaa ama joojisaa hydroxyacid oxidase 1 (HAO1) mRNA, waxay yaraynaysaa heerka glycolate oxidase ee beerka, ka dibna waxay isticmaashaa substrate-ka looga baahan yahay soo saarista oxalate, yaraynta wax soo saarka oxalate si loo xakameeyo horumarka cudurka ee bukaanka iyo hagaajinta calaamadaha cudurka.
(14) Viltepso
Shirkadda: Waxaa soo saartay NS Pharma, oo hoos timaada Nippon Shinyaku.
Waqtiga suuqa: Waxaa ogolaatay FDA suuqgeyntooda Agoosto 2020.
Tilmaamaha: Daawaynta Duchenne muscular dystrophy (DMD) oo leh exon 53 oo ka boodaya isbeddelka hidda-wadaha DMD.
Faallooyinka: Viltepso waa daawada oligonucleotide antisense taas oo ku xidhi karta booska exon 53 ee pre-mRNA ee hidda-wadaha DMD, taasoo keenaysa qayb ka mid ah exon 53 in laga saaro ka dib samaynta mRNA baaluq, taas oo qayb ahaan hagaajinaysa qaabka akhriska mRNA Sanduuqa wuxuu caawiyaa bukaanada si ay u soo saaraan qaar ka mid ah noocyada shaqada ee borotiinka ee dystrophin ee caadiga ah.
(15) Amondys 45
Shirkadda: Waxaa soo saartay Sarepta Therapeutics.
Waqtiga suuqa: Waxaa ogolaatay FDA suuqgeyntooda Febraayo 2021.
Tilmaamaha: Daawaynta Duchenne muscular dystrophy (DMD) oo leh exon 45 oo ka boodaya isbeddelka hidda-wadaha ee hiddaha DMD.
Fikradaha: Amondys 45 waa daawada oligonucleotide antisense, oligonucleotide antisense waxay ku xidhi kartaa booska exon 45 ee pre-mRNA ee hiddo-wadaha DMD, taasoo keentay in qaybta exon 45 la xannibo ka dib samaynta mRNA Excision, taas oo qayb ahaan hagaajinaysa qaab-dhismeedka borotiinka mRNA, kaas oo gacan ka geysanaya hagaajinta qaab-dhismeedka borotiinka. hagaajinta calaamadaha bukaanka.
(16) Amvuttra (vutrisiran)
Shirkadda: Waxaa soo saartay Alnylam Pharmaceuticals.
Waqtiga suuqa: Waxaa ogolaatay FDA suuqgeyntooda Juun 2022.
Tilmaamaha: Daawaynta amyloidosis transthyretin ee la iska dhaxlo oo leh polyneuropathy (hATTR-PN) ee dadka waaweyn.
Fiiro gaar ah: Amvuttra (Vutrisiran) waa daawada siRNA ee bar-tilmaameedsanaysa transthyretin (ATTR) mRNA, oo lagu maamulo cirbadaynta subcutaneous.Vutrisiran waxay ku salaysan tahay Alnylam's Enhanced Stability Chemistry (ESC) -GalNAc nashqadeynta madal gaarsiinta oo leh awood korodhay iyo xasilloonida dheef-shiid kiimikaadka.Oggolaanshaha daawadu waxay ku salaysan tahay xogta 9-bilood ee daraasaddeeda caafimaad ee Wajiga III (HELIOS-A), natiijooyinka guud waxay muujinayaan in daawadu ay hagaajisay calaamadaha hATTR-PN, iyo in ka badan 50% xaaladda bukaannada ayaa laga beddelay ama laga joojiyay inay ka sii daraan.
4. Daawooyinka kale ee daaweynta hidda-wadaha
(1) Rexin-G
Shirkadda: Waxaa soo saartay Epeius Biotech.
Waqtiga suuqa: Sannadkii 2005, waxaa loo oggolaaday suuqgeyntiisa Maamulka Cuntada iyo Dawooyinka ee Filibiin (BFAD).
Tilmaamaha: Daawaynta kansarrada horumaray ee u adkaysta daaweynta kiimikaad.
Xusuusin: Rexin-G waa cirbad nanoparticle ah oo hiddo-wadaha ku raran yahay.Waxay soo bandhigaysaa hidda-wadaha mutant G1 cyclin ee unugyada bartilmaameedka ah iyada oo loo marayo vector retroviral si ay si gaar ah u disho burooyinka adag.Habka maamulka waa faleebo xididka.Sida dawada buro-bartilmaameed ah oo si firfircoon u raadisa oo burburisa unugyada kansarka dheef-shiid kiimikaadka, waxay leedahay saameyn daweyn gaar ah bukaanada ku guul darreystay daawooyinka kale ee kansarka, oo ay ku jiraan bayooloji la beegsaday.
(2) Neovasculgen
Shirkad: Waxaa soo saaray Machadka Unugyada Asalka ee Human.
Waqtiga liiska: Waxaa loo oggolaaday liiska Ruushka Diseembar 7, 2011, ka dibna laga bilaabay Ukraine 2013.
Tilmaamaha: loogu talagalay daaweynta cudurrada xididdada xididada xididada xididada, oo ay ku jiraan ischemia addimada daran.
Xusuusin: Neovasculgen waa daawayn hidde-side ku salaysan plasmids DNA.Xakamaynta korriinka xididdada xididdada dhiigga (VEGF) 165 ayaa lagu dhisay laf-dhabarka plasmid waxaana lagu shubaa bukaannada.
(3) Collategene
Shirkadda: Waxaa si wadajir ah u horumariyay Jaamacadda Osaka iyo shirkadaha raasumaalka ah.
Waqtiga suuqa: Waxaa ogolaatay Wasaaradda Caafimaadka, Shaqada iyo Daryeelka ee Japan Agoosto 2019.
Tilmaamaha: Daaweynta ischemia hoose ee muhiimka ah.
Faallooyinka: Collategene waa daawaynta hidda-sidaha Plasmid-ku-salaysan, daawadii ugu horreysay ee daawaynta hidde-sidaha gudaha ee ay soo saarto AnGes, shirkad daawaynta hidda-wadaha ee Japan.Qaybta ugu muhiimsan ee dawadani waa plasmid qaawan oo ka kooban qodobka koritaanka hepatocyte ee bini'aadamka (HGF).Haddii daawada lagu duro murqaha lugaha hoose, HGF ee la muujiyay ayaa kor u qaadi doonta samaynta xididdada cusub ee dhiigga ee agagaarka xididdada dhiigga ee xiran.Tijaabooyin caafimaad ayaa xaqiijiyay saamaynta ay ku leedahay hagaajinta boogaha.
Sidee Foregene u caawin kartaa horumarinta daaweynta hidda-wadaha?
Waxaan ka caawinnaa badbaadinta waqtiga baaritaanka baarista baaxad weyn, marxaladda hore ee horumarinta daroogada siRNA.
Faahfaahin dheeraad ah booqo:
https://www.foreivd.com/cell-direct-rt-qpcr-kit-direct-rt-qpcr-series/
Waqtiga boostada: Dec-27-2022